WASHINGTON — If you use eyedrops, check out this recall announcement involving certain eye care products found at Walmart and CVS. They've been recalled for a possible lack of sterility. According to the FDA, unsterile eye products can potentially cause eye infections or related harm.
The products involved are:
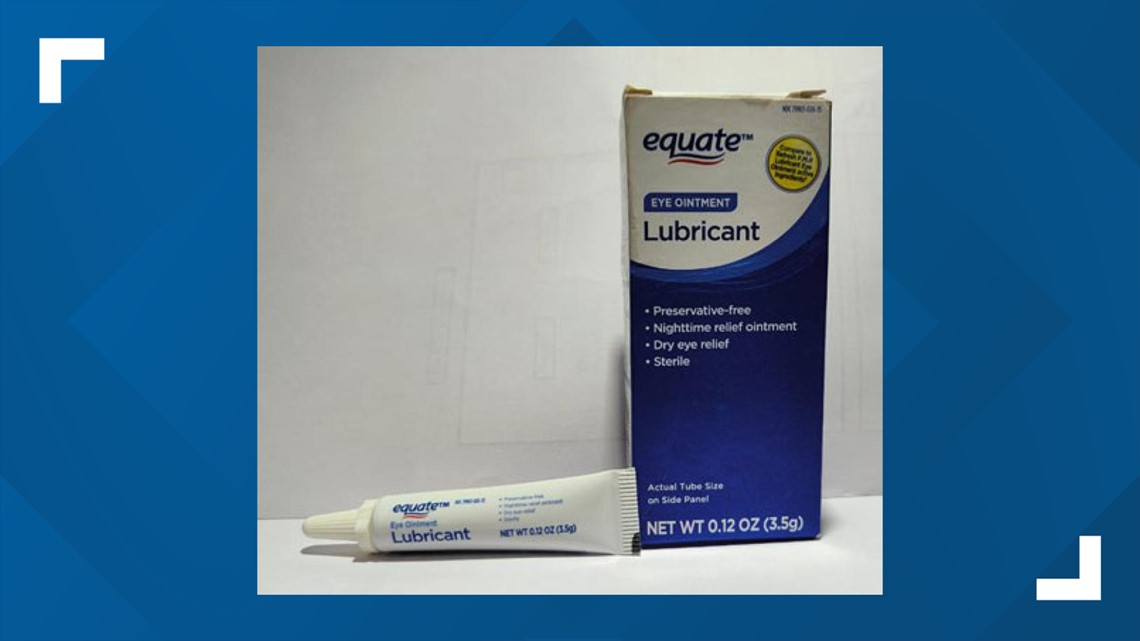
- Equate Lubricant Eye Ointment
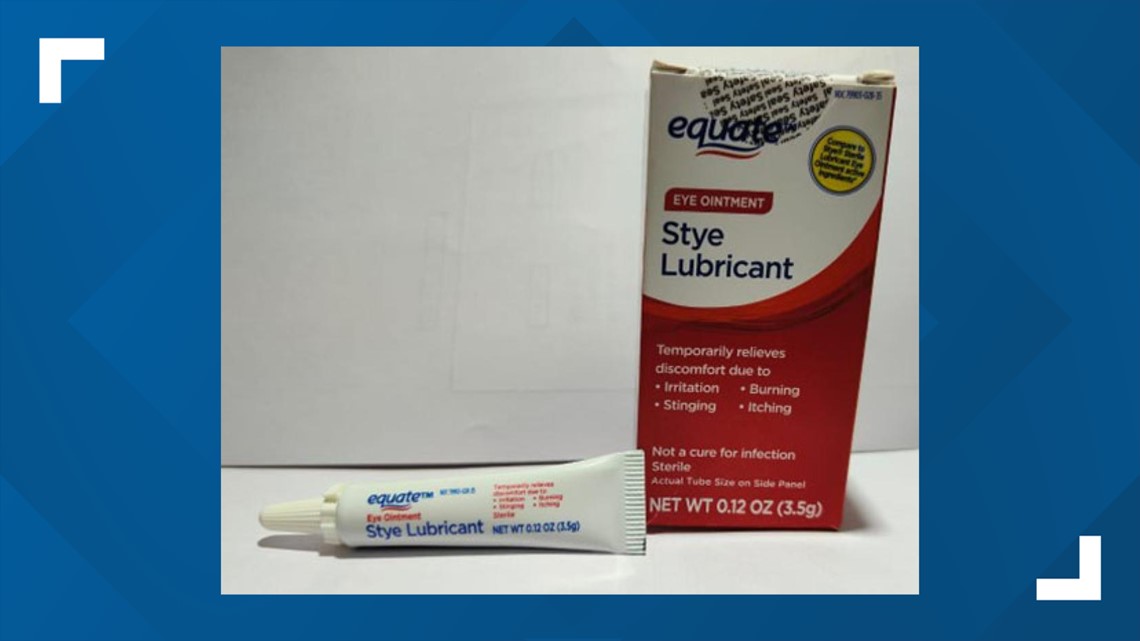
- Equate Stye Lubricant Eye Ointment
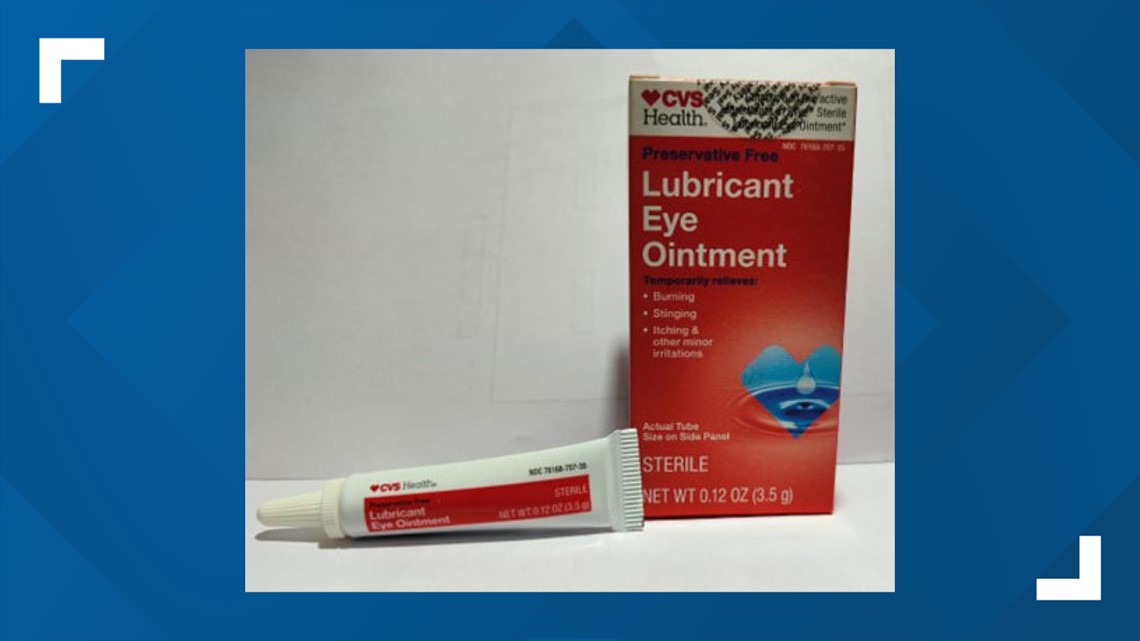
- CVS Health Lubricant Eye Ointment
- AACE Lubricant PM Ointment
Not all packages are affected. Under this current recall, the following products are affected:
- Equate Lubricant Eye Ointment
3.5 gram tube, packaged in cardboard box with UPC Code 681131395298
NDC 79903-026-35
Lot #/Exp Date:
A2E01/Apr24
A2L05/Nov24
A2B01/Jan25
A3C01/Feb25
A3H05/Jul25 - Equate Stye Lubricant Eye Ointment
3.5 gram tube, packaged in cardboard box with UPC Code 681131395304
NDC 79903-028-35
Lot #/Exp Date:
A2D08/Mar-24
A2F02/May-24
A2I03/Aug-24
A2L03/Nov-24
A2L04/Nov-24
A3C03/Feb-25
A3C05/Feb-25
A3H01/Jul-25
A3H03/Jul-25 - CVS Health Lubricant Eye Ointment
3.5 gram tube, packaged in cardboard box with UPC Code 050428634141
NDC 76168-707-35
Lot #/Exp Date:
A2F03/May-24
A2I02/Aug-24
A2L02/Nov-24
A3C04/Feb-25
A3H04Jul-25 - AACE Pharmaceuticals Lubricant PM Ointment
3.5 gram tube, packaged in cardboard box with UPC Code 371406124356
NDC 71406-124-35
Lot #/Exp Date:
A2G01/Jun-24
A2G02/Jun-24
A3F08/May-25
A3F09/May-25
A3J17/Sep-25
A3J18/Sep-25
The products were distributed nationwide to wholesalers and retailers. There have been no reports of injuries or illnesses through February 16, 2024.
If you have any of these products, you should stop using it and return it to the place of purchase.
The FDA says users with questions regarding this recall can contact Brassica Pharma Pvt. Ltd. at +1 833-225-9564 or info@brassicapharma.com. and contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Have a problem with these products? The FDA advises:
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178